Step 1 - Register Study / Obtain Approvals
NIH/NHGRI Data Sharing Policy Compliance
Institutions are responsible for assuring, through an Institutional Certification, that plans for the submission of large-scale human genomic data meet the expectations of the Genomic Data Sharing Policy.
To deposit large-scale, individual-level data into AnVIL, data submitters must follow the steps below.
1.1. Obtain approval
1.2. Register study
1.3. Apply to AnVIL
1.1. Obtain Approval
Working from an NIH grant?
If your Funding Opportunity Announcement (FOA) explicitly states that grantees are expected to share data in the AnVIL, you have this covered by default.
NHGRI-funded researcher?
For other work, including grants supported by Parent FOAs where data deposition in a certain repository isn’t described, PIs should discuss submission to AnVIL with your Program Director (PD). If you are an Intramural Researcher, discuss with your Scientific Director (SD). If the PD/SD agrees with submission of data to AnVIL, work with the NHGRI Genomic Program Administrator (GPA) to determine the appropriate place to register your study (see more on registration below).
NIH-funded researcher?
If your research is funded by another NIH Institute (not NHGRI), submit a request for the AnVIL to host your dataset by completing the AnVIL Dataset Onboarding Application form. Once AnVIL has agreed to host your study, contact your NIH Institute’s GPA (if you don't know your GPA, refer to this list) to determine the appropriate place to register your study (see more on registration below).
Not working from an NIH grant?
If your research is not funded by the NIH, identify an institute whose mission your research is most closely aligned with that is willing to sponsor your data submission.
At the same time, submit a request for AnVIL to host your dataset by completing the AnVIL Dataset Onboarding Application form. Once both have agreed to host your study, work with the GPA of the NIH Institute that agreed to sponsor your data submission to determine the appropriate place to register your study (see more on registration below).
1.2. Register study
Where you register your data and who will advise you of next steps depend on what kind of data you are generating and how your study is funded.
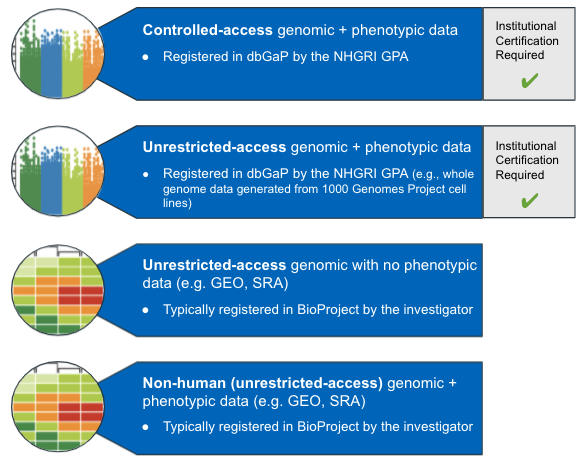
Generating human data? Register with dbGaP
See this link for more details about dbGaP and how to register your study data.
NHGRI-funded researcher?
- Contact the NHGRI Genomic Program Administrator (GPA).
- For information on how to register a dataset with NHGRI, see here.
- The GPA will request information from you to register your study.
NIH-funded researcher?
- Contact your Institutes and Centers GPA (if you don't know your GPA, refer to this list).
- The GPA will request information from you to register your study.
- Ask the GPA to select "AnVIL" for the External Data Repository field during registration.
Non-NIH-funded?
- Work with the GPA of your sponsoring NIH Institute.
- The GPA will lead you through the study registration process.
- Ask the GPA to select "AnVIL" for the External Data Repository field during registration.
Non-human data?
If you are working with non-human data, you will need to register with an NCBI repository such as GEO or SRA.
1.3. Apply to AnVIL
Prospective AnVIL data submitters should complete the AnVIL Dataset Onboarding Application for review by the AnVIL leadership committee.
Before you apply
Note that you will need the phsID from dbGap (step 1.2 above) and Access Rrstrictions (i.e., Data Use limitations/consent groups) to complete the application.
Access Restrictions
Known Data Use Limitations (DUL) - the list of requirements for gaining access and using the data - need to be clearly defined by the data depositor. This is completed via the NIH Institutional Certification form signed by the submitter's institutional official and provided directly to the Genomic Program Administrator for the IC (i.e,. NHGRI).
You should include your DULs as part of the AnVIL onboarding application. Consent information, along with other information listed in this spreadsheet, are documented in DUOS. Once ingested into TDR, DUOS will handle all protocols for gaining access.